- Home
- For Researchers & Academics
- Core Facilities
Steve N. Caritis Magee Obstetric Maternal & Infant (MOMI) Database and Biobank

Created to advance scientific understanding of maternal and infant health, the Steve N. Caritis Magee Obstetric Maternal & Infant (MOMI) Database and Biobank is one of the largest and most comprehensive resources of its kind. With a scientific focus on the quality and reliability of the specimens, the MOMI Database and Biobank collects obstetric biological materials along with annotated clinical information via a rigorous process for quality control. Our unique, web-based inventory system tracks our biological specimens, linking them to clinical data behind a secure, HIPAA compliant server.
These specimens and data are designed to assist researchers in a range of topics. A few examples include:
- The causes and prevention of complications of pregnancy
- The effectiveness and safety of medications during pregnancy
- The effectiveness of vaccines and how they impact maternal and infant immunity later in life
- The immunology of pregnancy and response to microbial infections
- How pregnancy affects women’s and infants’ health across the lifespan
- Opioid and drug use during pregnancy and the downstream effects on future generations
Publications
Database
The MOMI Database is comprised of clinical data from more than 300,000 deliveries, adding clinical information from more than 15,000 deliveries each year. MOMI contains all discrete data documented in the electronic health records relevant to obstetric care.
The upgraded version of MOMI, launched summer 2025, entails access to rich obstetric data beyond the previous flat file, extending to 13 delivery hospitals and inclusive of all discrete documentation in the electronic health record for obstetric patients. MOMI now leverages UPMC’s clinical and operational data warehouse and includes all variables in the EHR. Therefore, there is not an all-inclusive codebook. Instead, the MOMI Team works with investigators to assemble the appropriate data set, often using the most frequently requested, predefined variables related to delivery.
Upgraded Example MOMI Codebook
For information on additional variables, please reach out to the MOMI Team using the Request Form linked below.
Biobank Specimens
The Biobank includes:
- Specimens from all trimesters and from labor and delivery.
- We now have more than 250,000 specimens related to 7,000+ deliveries.
- Over 7,000 demographically representative participants with approximately 1,000 additional pregnancies included each year.
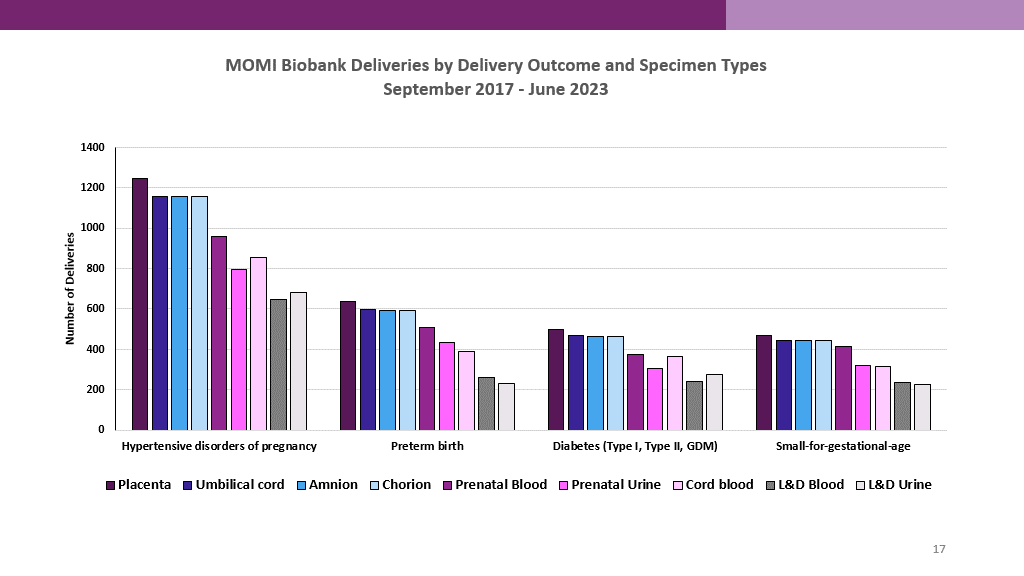
The Biobank specimens represent a variety of pregnancy complications including:
- Preeclampsia and hypertensive disorders of pregnancy
- Spontaneous and indicated preterm birth
- Fetal growth restriction
- Advanced maternal age (35+)
- Perinatal infections
- Smoking and substance use during pregnancy
- Obesity before and during pregnancy
- Gestational and pre-gestational diabetes
Banked specimens include:
- Longitudinally collected maternal blood
- Maternal and fetal blood at delivery
- Longitudinally collected maternal urine
- Tissue biopsies, currently including:
- Umbilical cord: snap frozen, placed in RNAlater, or formalin fixed
- Placenta, including villous tissue in RNAlater: snap frozen, OCT-embedded, and formalin-fixed and paraffin-embedded
- Placental membranes: Amnion and chorion collected separately for RNAlater and snap freezing. Membranes rolled together for formalin-fixation and paraffin embedding.
Quality Control
Consistent attention to quality control is a hallmark of all MOMI services. The time between specimen collection and processing is minimized and closely assessed according to the specimen type, to assure stability. The laboratory is directly adjacent to the collection site at UPMC Magee- Womens Hospital, one of the largest facilities of its kind in the United States. MOMI’s obstetric specimen procurement unit is physically located within the hospital’s labor and delivery suite.
Services Offered
MOMI’s team is able to expand its services based on research needs. For example, the Biobank also conducts prospective tissue collection and is approved for additional pregnancy and delivery specimens. The MOMI Biobank can work with principal investigators to provide customized specimens to strengthen research initiatives. In addition to specimen collection, MOMI offers honest broker services and can prepare sections and/or stain slides. We can also provide referrals for other studies. All users must agree to the terms set forth in the MOMI Data and Biological Specimen User Agreement, as well as any applicable contractual agreements. Specimens cannot be disbursed prior to appropriate approvals.
We offer:
- Honest Broker and IRB services
- Research Histology
- Prospective tissue banking and collection
- In-person and/or mailed study referrals
To inquire or to submit a request for MOMI specimens and/or clinical data, please complete the MOMI Request Form.

If you have an active or outstanding request, please use this addendum form for any additions or changes to your original submission. Additional charges may be applied.
If you’re an industry member interested in partnering with or utilizing the MOMI Biobank please reach out to Lara Lemon at lemonl@upmc.edu.
What to expect as an investigator.
The MOMI team will reach out to you after our monthly data governance meets to review your request. If approved, to expedite your data/sample acquisition, please begin to familiarize yourself with institutional processes and initiate communication to facilitate contracting and accounting:
| Request to Fulfillment Process |
|---|
| 1. Submit Request Form 2. Requests reviewed the last week of each month 3. MOMI Team will reach out to finalize request upon approval 4. Administration Documents Needed from PI: - IRB Approval Letter - IRB Protocol detailing use of MOMI - Honest Broker Form - Purchase Order # (for budget) - Shipping address & FedEx account #, if Biospecimen request 5. Collaborative Documents: - Budget - Execution of Material Transfer Agreement/Data Use Agreement (Drafted by MOMI contract specialist) 6. Data/Samples Shared |
Resources for Award Applications and IRB Submissions
Including MOMI in your application
IRB Submission Resources:
MOMI Biobank Participant Consent
Honest Broker Form (example)
Exemption Form, Secondary Research with Data and/or Specimens (example)
Grant Submission Resources:
Boilerplate Facilities Language
Location and Contact Information
MOMI Database and Biobank Core
Magee-Womens Research Institute
204 Craft Avenue
Pittsburgh, PA 15213
MOMI Data Manager
Jeannette Wellman
E: wellmanjl2@upmc.edu
Be the First to Know
Get the latest research, news, events, and more delivered to your inbox.